Background: Exosomes as Therapeutics
What are Exosomes?
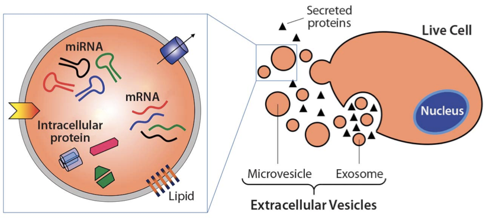
Exosomes are nanosized, extracellular vesicles secreted by a variety of cells, including stem cells. They carry important signals from one cell to another in the form of “cargoes,” such as microRNAs or messenger RNAs. These can induce changes in the behavior of the recipient cells, including reducing a diseased cell behavior or “phenotype,” including inflammation.
Exosome treatments circumvent many of the challenges and concerns associated with stem cell transplantation (immunorejection, tumorigenesis, limited survival/assimilation).
They allow for selective control over what contents are delivered my measuring or modifying their cargoes.
Exosomes are blood-brain barrier permeable and can migrate between cells in ways that implanted stem cells can’t. This allows for unique advantages in drug delivery.
Exosomes can be engineered to target specific cell types.
01-22-Das-lab-Exosomes-article-figure1.png
From Wikipedia: “Exosomes are cell-derived vesicles that are present in many and perhaps all biological fluids, including blood, urine, and cultured medium of cell cultures. Exosomes contain various molecular constituents of their cell of origin, including proteins and RNA. It is becoming increasingly clear that exosomes have specialized functions and play a key role in, for example, coagulation, intercellular signaling, and waste management.”
Exosome Isolation Process from Stem Cells
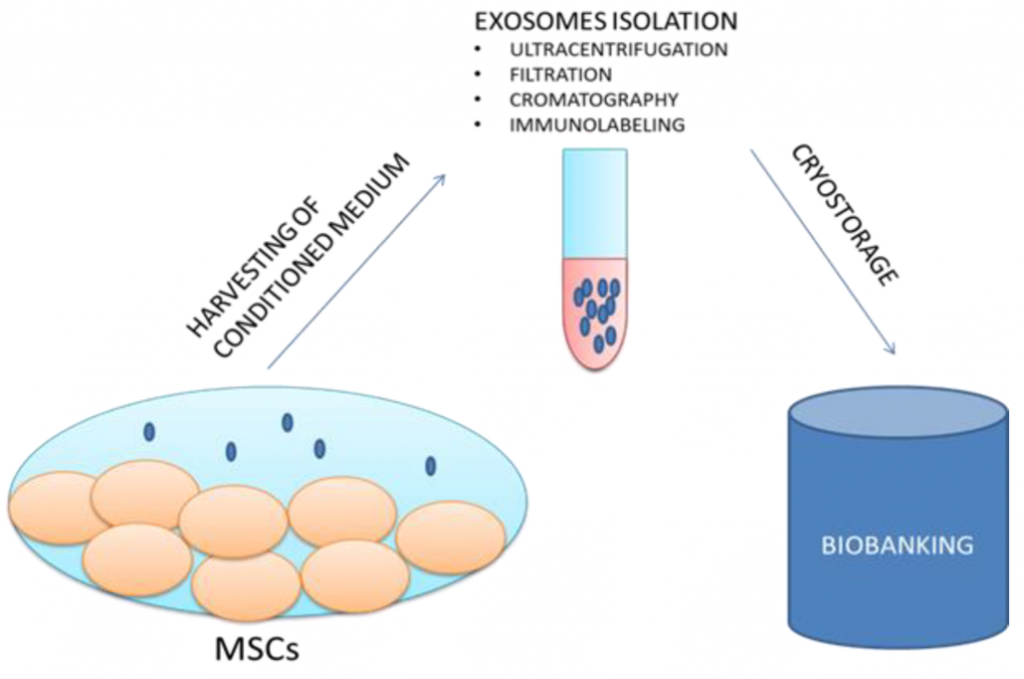
Exosome isolation from human stem cells:
- Stem cells are cultured in sterile plate
- Secreted exosomes are harvested in conditioned medium
- Exosomes are isolated and purified
- Exosomes are stored for preservation
Current Study: Neural stem cell exosomes for human aging-associated neuroinflammation and cognitive decline
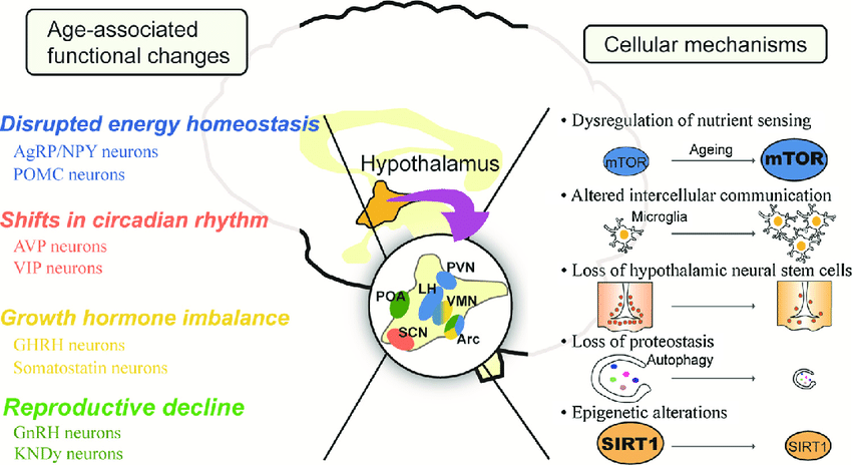
Exosome-based Therapy for Hypothalamic Inflammation (HI) and Other Brain Pathologies
- Hypothalamic inflammation (HI) is associated with aging, metabolic disease and obesity through a variety of neuroendocrine pathways
- Neural stem cells (NSCs) exert a protective and inflammation-suppressant role on the brain in aged mice
a. Found to be mediated by stem cell-derived exosomes and their therapeutic contents
b. Protective effect of NSCs has been restored experimentally through exogenous exosome application - There is evidence that NSCs and their exosomes can also promote healing of damaged neurovascular tissue in traumatic brain injury and stroke
a. NSC exosomes contain a number of factors that promote growth and tissue-healing - NSC-derived exosomes are being tested for patient applications to ameliorate aging-associated cognitive decline and dementia, as well as a variety of metabolic and neurodegenerative diseases in the brain
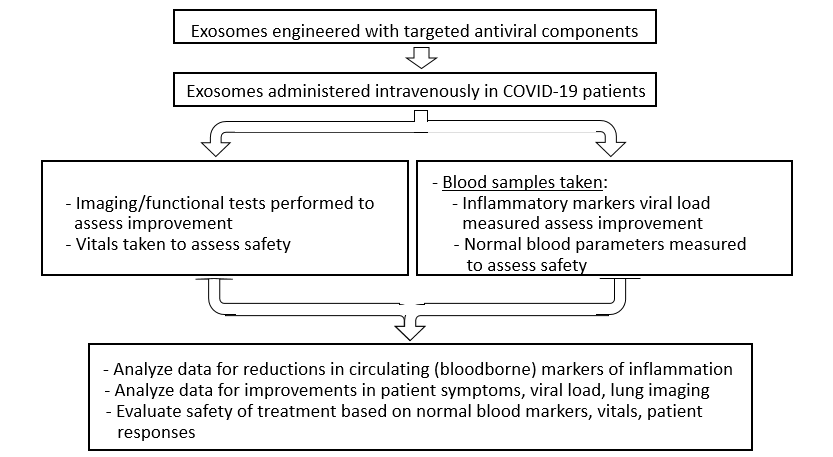
Experimental Design
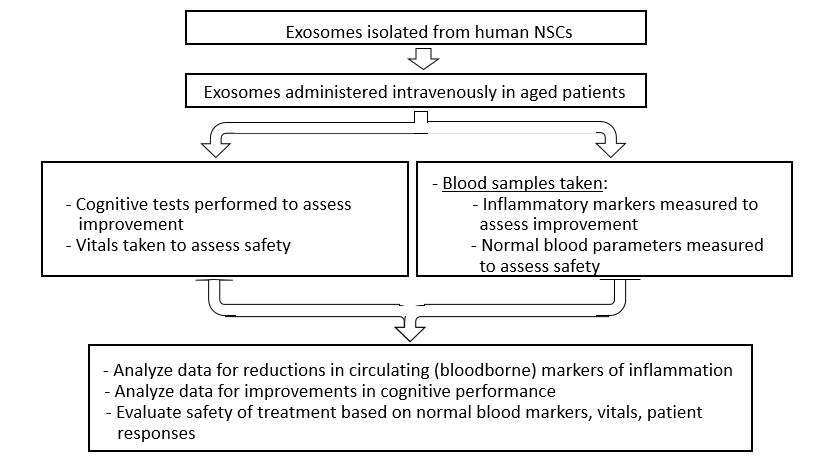
Summary Hypothesis of Effects
Current Study: Exosomal delivery of targeted antiviral and acute respiratory distress therapies for COVID-19
Exosome-based Therapy for COVID-19
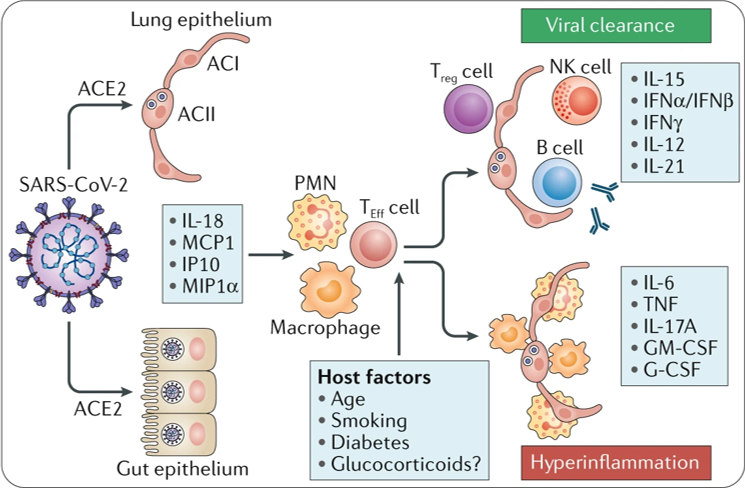
- COVID-19 progression is associated with a ‘cytokine storm,’ a significant elevation in inflammatory cytokines that are involved in acute respiratory distress syndrome (ARDS), as well as causing in some cases long-term damage to multiple organ systems.
- Exosomes can be used to deliver contents that suppress the cytokine storm, thereby reducing respiratory distress and organ damage.
- We are also engineering specific viral-targeted constructs that can be delivered by exosomes to cells in order to ‘deactivate’ the SARS-Cov-2 virus that causes COVID-19.
Experimental Design
Summary Hypothesis of Effects
iPSC-based Platform Development of Cell Lineage-Specific Therapies
- Human induced pluripotent stem cells (iPSCs) are cells derived from human somatic tissues such as skin fibroblasts, which are then reprogrammed into stem cells
- iPSCs can be differentiated into a wide variety of cell types, each with their own unique properties and therapeutic potential. As such, they have incredible potential as a platform for the development of multiple therapies for human disease
- iPSCs can also be used to model disease states. If they are derived from patients with a known mutation or if a mutation is induced, the properties of specific cell types in the body derived from the parent iPSCs can then be explores to see how the disease affects them
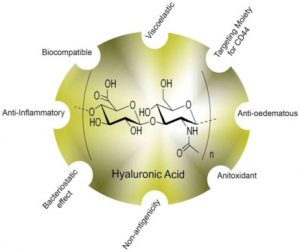
Hyaluronic acid (HA): a nano-carrier for exosome delivery
- Is a naturally-occurring macromolecule component of the brain extracellular matrix
- Is being studied as a gel matrix for stem cell delivery, tissue engineering, and brain repair after stroke
- Has been used experimentally to increase the stability and solubility of cancer drugs
- As a nanoemulsion, can be used to protect therapeutic exosomes for gradual release from delivery site at the nasal mucosa, into the brain
(Sources for images: Zhu Z, Yin-Min W, Jun Y. Hyaluronic acid: a versatile biomaterial in tissue engineering. Plast Aesthet Res. 2017;4(219-27)
Wickens JM, Alsaab HO, Kesharwani P, Bhise K, Amin MC, Tekade RK, Gupta U, Iyer AK. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug discovery today. 2017 Apr 1;22(4):665-80.
Shinde RL, Bharkad GP, Devarajan PV. Intranasal microemulsion for targeted nose to brain delivery in neurocysticercosis: role of docosahexaenoic acid. European Journal of Pharmaceutics and Biopharmaceutics. 2015 Oct 1;96:363-79.)
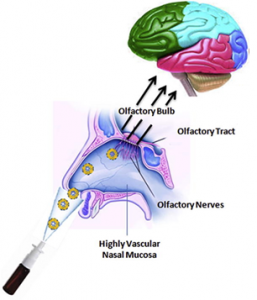
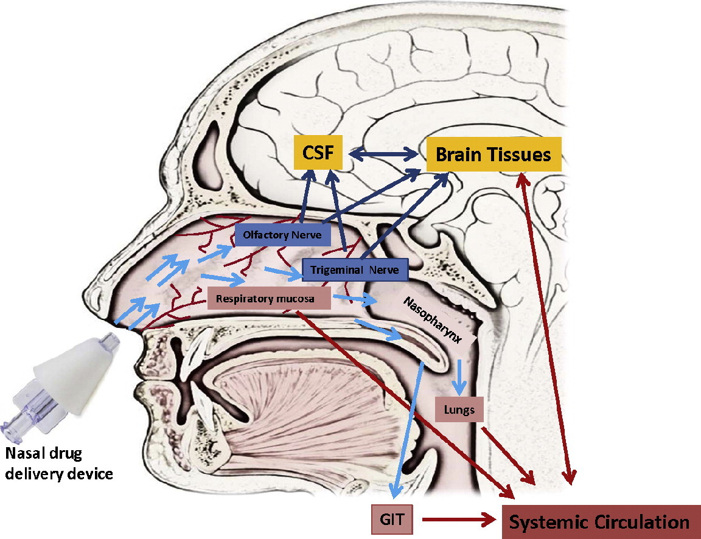
Intranasal Delivery of Therapeutics for CNS Pathologies
- Intranasal delivery involves delivering a therapeutic agent through the nostrils into the nasal cavity, for uptake into the brain
- This approach carries several advantages for enhanced delivery, as well as avoiding effects in non-brain organs when delivery to these (as through the bloodstream) is not desired
- This approach allows delivery in a way that is less invasive for many patients, and more convenient for at-home delivery
- Several components can be added to enhance nose-to-brain uptake
Biomaterial-assisted Sublingual Delivery of Therapeutics
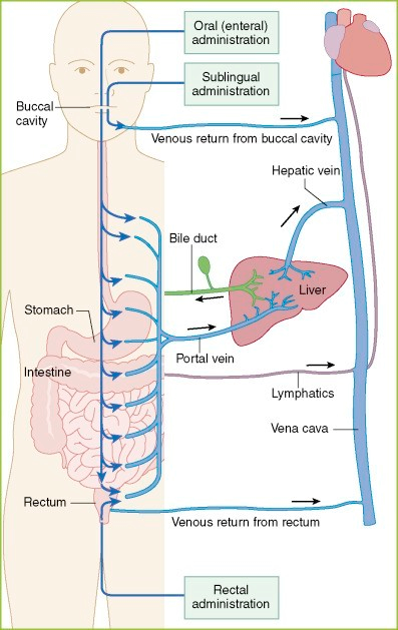
- Biomaterial film matrices are being developed that can accommodate delivery of therapeutic agents through the sublingual/buccal route, under the tongue
- Sublingual delivery avoids first-pass metabolism through the gut and liver, providing more direct delivery to the bloodstream than oral administration
- This approach allows delivery to the bloodstream in a way that is less invasive for many patients than intravenous injection, and more convenient for at-home delivery
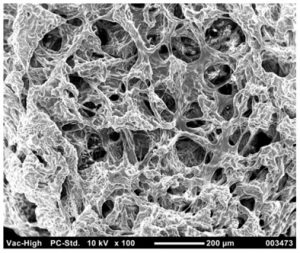
3D Culture System
StemXO is actively developing optimized systems for 3-dimensional cell culture, both for organoid modeling and for scaled cell-based therapy manufacturing.
Traditional cell culture approaches involve the expansion of cells in 2-dimensional, adherent ‘monolayers.’ While this approach simplifies the examination of cell morphology and the addition of growth factors and harvesting of secreted products, it also places cells in an unnatural growth environment which does not accurately model their function in physiological settings.
StemXO’s proprietary cell culture system incorporates novel, state-of-the art innovations to culture stem and other cells, representing a variety of organ tissues, in 3D organoids in an efficient manner that allows for effective experimental modeling and high throughput drug/toxicology screening.
From the therapeutic standpoint, our innovative design also allows for significantly increased scalability for cell and cell-derived exosome therapies compared with conventional culture and expansion methods.
StemXO is also investigating in parallel approaches to engineer functional, implantable human organ tissues from 3D scaffolding templates, to address the vast unmet need for patients awaiting viable implant alternatives for liver, kidney and heart, as well as providing improved skin grafts and integrated peripheral nerve bridges.
Automated GMP Culture System
StemXO, in collaboration with our affiliate Robowind (-insert hyperlink here-), is developing a fully automated, GMP-compliant closed system for compact and scaleable cell culture and secretory factor purification.
The efficient production of expanded cell lines for research as well as clinical therapeutics is currently limited by the costs of maintaining personnel for regular passaging, maintenance and monitoring. The multi-step transfer of cultures between incubator and sterile culture hood also introduces the possibility for the risk of contamination or variability in the growth environment.
StemXO’s automated culture system will revolutionize the cell manufacturing process by eliminating the need for direct investigator participation in a majority of the culture process, while providing a medium for monitoring culture conditions through high-resolution camera imaging.
As our system is closed/sterile, fully internal and GMP-compliant, it will also eliminate the need for often costly use of a GMP facility for biological manufacturing.
